Atoms Contain Mostly Empty Space
How much of an atom is empty space?
Very nearly all of it. Permit'southward take a look at an cantlet of hydrogen to see how empty it really is. Of grade, this diagram isn't drawn to scale...
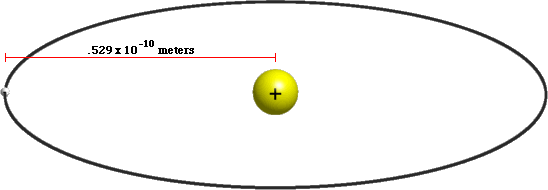
A hydrogen atom is fabricated from a single proton that'due south circled by a single electron. How large is a hydrogen atom? The radius of a hydrogen atom is known equally the Bohr Radius, which is equal to .529 × ten-x meters. That ways that a hydrogen atom has a volume of near 6.2 × x-31 cubic meters.
How big is the proton at the center of a hydrogen atom? Recent studies indicate that protons have a radius of most .84 × 10-15 meters, giving them a volume of most 2.v × 10-45 cubic meters.
We need to do a niggling more math to find out how much of a hydrogen atom is empty infinite:
Per centum Full = 100 × (Volume Filled / Full Volume)
Per centum Full = 100 × (2.5 × ten-45 10003 / half-dozen.2 × 10-31 10003)
Pct Full = 100 × (iv × x-15)
Percent Full = four × 10-xiii %
Percent Full = 0.0000000000004%
If 0.0000000000004% of a hydrogen atom is full, then the rest of it must exist empty:
Percent Empty = 100% - Pct Full
Percent Empty = 100% - 0.0000000000004%
Percent Empty = 99.9999999999996%
A hydrogen atom is about 99.9999999999996% empty space. Put another way, if a hydrogen atom were the size of the earth, the proton at its centre would be nigh 200 meters (600 feet) beyond. While I wouldn't want something that big landing on my head, it's tiny compared to the size of the earth.
Atoms Contain Mostly Empty Space,
Source: https://education.jlab.org/qa/how-much-of-an-atom-is-empty-space.html
Posted by: jacobsthujered.blogspot.com


0 Response to "Atoms Contain Mostly Empty Space"
Post a Comment