What Is The Conjugate Base
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid donates a proton (H+ ) to a base—in other words, it is a base of operations with a hydrogen ion added to information technology, every bit in the contrary reaction it loses a hydrogen ion. On the other paw, a conjugate base is what is left over after an acid has donated a proton during a chemical reaction. Hence, a conjugate base is a species formed by the removal of a proton from an acid, as in the opposite reaction information technology is able to proceeds a hydrogen ion.[1] Because some acids are capable of releasing multiple protons, the cohabit base of an acrid may itself exist acidic.
In summary, this can be represented as the post-obit chemical reaction:


Johannes Nicolaus Brønsted and Martin Lowry introduced the Brønsted–Lowry theory, which proposed that any compound that can transfer a proton to whatever other compound is an acid, and the compound that accepts the proton is a base. A proton is a nuclear particle with a unit positive electrical charge; information technology is represented past the symbol H+ considering information technology constitutes the nucleus of a hydrogen atom,[two] that is, a hydrogen cation.
A cation tin be a conjugate acid, and an anion tin can be a conjugate base, depending on which substance is involved and which acid–base of operations theory is the viewpoint. The simplest anion which can exist a conjugate base is the solvated electron whose cohabit acid is the atomic hydrogen.
Acrid–base reactions [edit]
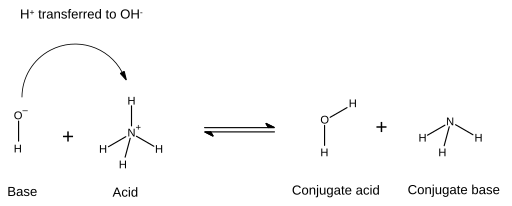
In an acid–base of operations reaction, an acid plus a base of operations reacts to form a conjugate base of operations plus a conjugate acrid. The acid loses a proton and the base of operations gains a proton. In chemical diagrams which illustrate this, the new bond formed between the base of operations and the proton is shown by an arrow that conventionally starts on an electron pair from the base of operations and whose arrow-head ends at the hydrogen ion (proton) that will be transferred:
In this case, the water molecule is the conjugate acid of the hydroxide ion afterward the latter received the hydrogen ion donated by ammonium. On the other hand, ammonia is the conjugate base for the acid ammonium later ammonium has donated a hydrogen ion and produced the h2o molecule. Also, OH− can be considered as the conjugate base of operations of H
2 O, since the h2o molecule donates a proton to give NH +
4 in the opposite reaction. The terms "acrid", "base of operations", "cohabit acid", and "conjugate base" are not fixed for a certain chemical species but are interchangeable co-ordinate to the reaction taking place.
Force of conjugates [edit]
The strength of a conjugate acid is direct proportional to its dissociation abiding. If a conjugate acid is stiff, its dissociation will take a higher equilibrium constant and the products of the reaction will be favored. The forcefulness of a conjugate base can be seen as the trend of the species to "pull" hydrogen protons towards itself. If a cohabit base of operations is classified every bit strong, it volition "hold on" to the hydrogen proton when in solution and its acid will not dissociate.
If a species is classified every bit a strong acid, its conjugate base volition be weak.[3] An example of this case would be the dissociation of hydrochloric acid HCl in water. Since HCl is a strong acid (it dissociates to a keen extent), its conjugate base of operations (Cl −
) will be a weak cohabit base of operations. Therefore, in this organization, most H +
will exist in the form of a hydronium ion H
3 O +
instead of fastened to a Cl− anion and the conjugate base will be weaker than a water molecule.
On the other hand, if a species is classified as a weak acid its conjugate base will not necessarily be a strong base. Consider that acetate, the conjugate base of acerb acid, has a base dissociation constant (Kb) of approximately 5.6×ten−10 , making information technology a weak base. In order for a species to accept a strong conjugate base it has to be a very weak acid, like h2o for case.
Identifying cohabit acid–base pairs [edit]
To identify the cohabit acid, look for the pair of compounds that are related. The acid–base of operations reaction can be viewed in a before and after sense. The before is the reactant side of the equation, the subsequently is the production side of the equation. The conjugate acid in the later on side of an equation gains a hydrogen ion, then in the before side of the equation the compound that has one less hydrogen ion of the conjugate acid is the base. The conjugate base of operations in the later on side of the equation lost a hydrogen ion, and then in the before side of the equation, the compound that has i more than hydrogen ion of the conjugate base is the acid.
Consider the following acrid–base reaction:
- HNO
3 + H
2 O → H
three O +
+ NO −
3
Nitric acid (HNO
iii ) is an acid because it donates a proton to the h2o molecule and its conjugate base is nitrate (NO −
3 ). The water molecule acts as a base because it receives the hydrogen cation (proton) and its conjugate acrid is the hydronium ion (H
3 O +
).
| Equation | Acid | Base of operations | Cohabit base | Cohabit acid |
|---|---|---|---|---|
| HClO 2 + H ii O → ClO − two + H 3 O + | HClO 2 | H 2 O | ClO − 2 | H three O + |
| ClO − + H 2 O → HClO + OH − | H 2 O | ClO − | OH − | HClO |
| HCl + H 2 PO − four → Cl − + H 3 PO 4 | HCl | H two PO − four | Cl − | H 3 PO 4 |
Applications [edit]
One use of conjugate acids and bases lies in buffering systems, which include a buffer solution. In a buffer, a weak acrid and its conjugate base (in the form of a salt), or a weak base and its conjugate acid, are used in order to limit the pH modify during a titration process. Buffers accept both organic and non-organic chemical applications. For example, besides buffers being used in lab processes, human claret acts as a buffer to maintain pH. The well-nigh of import buffer in our bloodstream is the carbonic acid-bicarbonate buffer, which prevents drastic pH changes when CO
2 is introduced. This functions as such:
Furthermore, here is a table of common buffers.
-
Buffering agent pKa Useful pH range Citric acid 3.13, iv.76, half-dozen.40 two.ane - 7.4 Acetic acid 4.eight 3.8 - five.8 KHtwoPO4 7.2 half dozen.2 - 8.ii CHES 9.3 eight.3–x.3 Borate ix.24 8.25 - x.25
A 2d common application with an organic compound would exist the production of a buffer with acetic acrid. If acetic acid, a weak acid with the formula CH
iii COOH, was made into a buffer solution, it would demand to be combined with its cohabit base of operations CH
3 COO −
in the form of a salt. The resulting mixture is called an acetate buffer, consisting of aqueous CH
3 COOH and aqueous CH
iii COONa. Acerb acid, along with many other weak acids, serve as useful components of buffers in different lab settings, each useful within their ain pH range.
Ringer's lactate solution is an example where the cohabit base of operations of an organic acrid, lactic acid, CH
three CH(OH)CO −
ii is combined with sodium, calcium and potassium cations and chloride anions in distilled water[iv] which together form a fluid which is isotonic in relation to human claret and is used for fluid resuscitation after claret loss due to trauma, surgery, or a burn down injury.[v]
Table of acids and their conjugate bases [edit]
Tabulated below are several examples of acids and their conjugate bases; notice how they differ past simply one proton (H+ ion). Acrid strength decreases and conjugate base strength increases downwards the tabular array.
| Acrid | Conjugate base |
|---|---|
| H 2 F + Fluoronium ion | HF Hydrogen fluoride |
| HCl Hydrochloric acid | Cl− Chloride ion |
| HiiAnd sofour Sulfuric acid | HSO − 4 Hydrogen sulfate ion (bisulfate ion) |
| HNO3 Nitric acrid | NO − 3 Nitrate ion |
| H3O+ Hydronium ion | H2O Water |
| HSO − iv Hydrogen sulfate ion | SO 2− four Sulfate ion |
| HthreePO4 Phosphoric acrid | H2PO − 4 Dihydrogen phosphate ion |
| CH3COOH Acetic acid | CH3COO− Acetate ion |
| HF Hydrofluoric acrid | F− Fluoride ion |
| H2CO3 Carbonic acrid | HCO − three Hydrogen carbonate ion |
| H2S Hydrosulfuric acid | HS− Hydrogen sulfide ion |
| H2PO − 4 Dihydrogen phosphate ion | HPO 2− 4 Hydrogen phosphate ion |
| NH + 4 Ammonium ion | NH3 Ammonia |
| H2O H2o (pH=seven) | OH− Hydroxide ion |
| HCO − 3 Hydrogencarbonate (bicarbonate) ion | CO 2− 3 Carbonate ion |
Tabular array of bases and their conjugate acids [edit]
In contrast, here is a tabular array of bases and their conjugate acids. Similarly, base strength decreases and conjugate acid strength increases downward the table.
| Base of operations | Conjugate acrid |
|---|---|
| C 2 H five NH 2 Ethylamine | C 2 H five NH + 3 Ethylammonium ion |
| CH 3 NH 2 Methylamine | CH iii NH + 3 Methylammonium ion |
| NH iii Ammonia | NH + 4 Ammonium ion |
| C 5 H 5 N Pyridine | C v H 6 Due north + Pyridinium |
| C half-dozen H 5 NH 2 Aniline | C 6 H five NH + 3 Phenylammonium ion |
| C half-dozen H v CO − ii Benzoate ion | C 6 H 6 CO ii Benzoic acid |
| F − Fluoride ion | HF Hydrogen fluoride |
| PO 3− 4 Phosphate ion | HPO 2− 4 Hydrogen phosphate ion |
| OH− Hydroxide ion | H2O Water (neutral, pH 7) |
| HCO − iii Bicarbonate | H ii CO 3 Carbonic acid |
| CO 2− three Carbonate ion | HCO − iii Bicarbonate |
| Br − Bromine | HBr Hydrogen bromide |
| HPO 2− 4 Hydrogen Phosphate | H 2 PO − 4 |
| Cl − Chloride ion | HCl Hydrogen chloride |
| H ii O Water | H three O + Hydronium ion |
| Nitrite ion | Nitrous acid |
See also [edit]
- Buffer solution
- Deprotonation
- Protonation
- Table salt (chemical science)
References [edit]
- ^ Zumdahl, Stephen S., & Zumdahl, Susan A. Chemistry. Houghton Mifflin, 2007, ISBN 0618713700
- ^ "Brønsted–Lowry theory | chemistry". Encyclopedia Britannica . Retrieved 25 February 2020.
- ^ "Strength of Cohabit Acids and Bases Chemistry Tutorial". www.ausetute.com.au . Retrieved 25 February 2020.
- ^ British national formulary: BNF 69 (69 ed.). British Medical Association. 2015. p. 683. ISBN9780857111562.
- ^ Pestana, Carlos (7 April 2020). Pestana's Surgery Notes (5th ed.). Kaplan Medical Examination Prep. pp. 4–5. ISBN978-1506254340.
External links [edit]
- MCAT General Chemistry Review - 10.4 Titration and Buffers
- The Pharmaceutics and Compounding Laboratory - Buffers and Buffer Capacity.
What Is The Conjugate Base,
Source: https://en.wikipedia.org/wiki/Conjugate_(acid-base_theory)
Posted by: jacobsthujered.blogspot.com






0 Response to "What Is The Conjugate Base"
Post a Comment